Development of vaccines against blood-stage P. Falciparum
Development of vaccines against blood-stage P. falciparum
The early days of the Draper group were focussed on the development of viral vectored vaccines targeting two major candidate blood-stage antigens from the human malaria parasite P. falciparum – MSP1 and AMA1. Our research showed effective T-cell and antibody immunogenicity in preclinical models, and was the basis for the Phase I/IIa clinical work that followed. Whilst neither of these two antigens were progressed further due to a lack of protective efficacy in the human challenge model, it allowed us to establish biomanufacturing and clinical know-how that underpins our work in experimental and translational medicine. These studies also provided an opportunity to better understand how candidate vaccines can induce immune responses in humans, as well as how these responses can be modulated by exposure to the parasite. Our vaccine development work subsequently focussed on identifying improved antigen targets within the blood-stage merozoite parasite. We have established various vaccine production platforms; including virus-like particles (VLP), protein-in-adjuvant, and mRNA, which enable us to generate and test a wide range of candidate vaccines.
We have also contributed significantly to the establishment of PfRH5 as the new leading antigen target in the P. falciparum merozoite. PfRH5 is an essential and highly conserved merozoite protein that interacts with its human receptor basigin on the surface or red blood cells during parasite invasion. We have shown that PfRH5 is highly susceptible to broadly-neutralising vaccine-induced antibodies, and we were able to demonstrate a first-generation PfRH5 vaccine can significantly reduce parasite growth in the blood following controlled human malaria infection (CHMI) challenge of vaccinated volunteers – a milestone for the blood-stage malaria vaccine field. Building on this success, we were the first to show that a PfRH5 is safe and efficacious in a double-blind, randomised, controlled Phase 2b trial in Burkinese children. This was the first demonstration of field efficacy of a blood-stage malaria vaccine.
Our current work continues via a two pronged approach. Firstly, to deepen our understanding of vaccine-induced antibody responses to PfRH5 in humans. This molecular understanding of how human antibodies can block the parasite (or in many cases can fail to block!) feeds back into our next-generation vaccine design strategies, allowing us to further develop and design improved and more potent PfRH5-based vaccine formulations. Secondly, we have developed new vaccine candidate, called “R78C”, based on the PfCyRPA and PfRIPR proteins. These two proteins form a complex with PfRH5 that is essential to erythrocyte invasion. We have shown pre-clinically that this new vaccine, when given in combination with PfRH5, is superior to PfRH5 alone. R78C has now completed a Phase I clinical study in the UK.
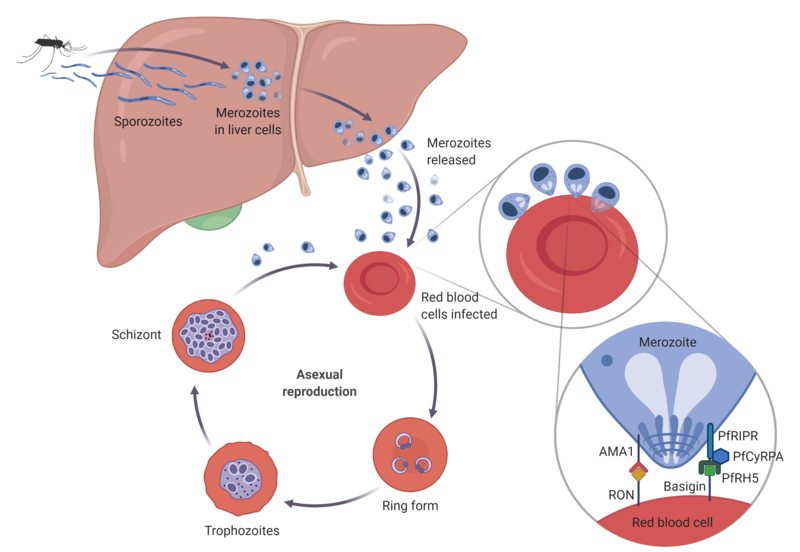
Blood-stage vaccine target antigens at the interface of P. falciparum and RBCs
For more information:
Douglas, Alexander D., et al. "The blood-stage malaria antigen PfRH5 is susceptible to vaccine-inducible cross-strain neutralizing antibody." Nature communications 2.1 (2011): 1-9.
Sheehy, Susanne H., et al. "Phase Ia clinical evaluation of the safety and immunogenicity of the Plasmodium falciparum blood-stage antigen AMA1 in ChAd63 and MVA vaccine vectors." PloS one 7.2 (2012): e31208.
Douglas, Alexander D., et al. "A PfRH5-based vaccine is efficacious against heterologous strain blood-stage Plasmodium falciparum infection in aotus monkeys." Cell host & microbe 17.1 (2015): 130-139.
Jin, Jing, et al. "Accelerating the clinical development of protein-based vaccines for malaria by efficient purification using a four amino acid C-terminal ‘C-tag’." International journal for parasitology 47.7 (2017): 435-446.
Payne, Ruth O., et al. "Human vaccination against RH5 induces neutralizing antimalarial antibodies that inhibit RH5 invasion complex interactions." JCI insight 2.21 (2017).
Minassian, Angela M., et al. "Reduced blood-stage malaria growth and immune correlates in humans following RH5 vaccination." Med (2021).
Williams, Barnabas G., et al. "Development of an improved blood-stage malaria vaccine targeting the essential RH5-CyRPA-RIPR invasion complex." Nature Communications 15.1 (2024): 4857.
Natama, Hamtandi M., et al. "Safety and efficacy of the blood-stage malaria vaccine RH5. 1/Matrix-M in Burkina Faso: interim results of a double-blind, randomised, controlled phase 2b trial in children." medRxiv (2024): 2024-10.


