Development of vaccines against blood-stage P. vivax
Development of vaccines against blood-stage P. vivax
is the second most common cause of malaria and the most geographically widespread. However, research into P. vivax has historically been neglected and lags behind that for P. falciparum. There are also important biological differences between P. vivax and P. falciparum, which limit the effectiveness of current malaria control measures. Unlike P. falciparum, P. vivax can remain dormant in the liver after the initial infection and these dormant parasites can recrudesce and cause repeated disease (relapses) months or even years after the initial infection.
During the blood-stage of the life cycle of P. vivax, the parasites invade immature red blood cells called reticulocytes, where they replicate to large numbers and cause the symptoms of malaria. Our efforts aim to design vaccines that prevent the invasion of red blood cells by the blood-stage parasite. The main candidate antigen for the development of a vaccine against P. vivax is the Duffy-binding protein (PvDBP). Through its interaction with the Duffy antigen receptor for chemokines (DARC/Fy) on the surface of reticulocytes, PvDBP plays an essential role in parasite invasion. A human gene polymorphism which results in lack of Duffy antigen expression on red blood cells (Duffy blood group negativity) is associated with natural protection against P. vivax infection. This gene polymorphism is commonly found in people from Sub-Saharan Africa and explains the low levels of P. vivax in this area. Blocking the interaction between PvDBP and DARC with antibodies induced by vaccination is thus a highly attractive strategy for vaccine development.
PvDBP can be divided into six regions, with the receptor (DARC) binding site mapped to a ~350 amino acid domain known as Region II (PvDBPII). We have tested two vaccines targeting PvDBPII for efficacy in clinical trials using controlled human malaria infection (CHMI). Our trials showed that the protein-in-adjuvant PvDBPII vaccine, when given in a delayed third dose regimen, was able to slow parasite growth in blood by about a half compared to unvaccinated volunteers during CHMI. The viral-vectored PvDBPII vaccine did not significantly affect parasite growth. See our News Item linked here!
Our current work on P. vivax is being conducted as part of the OptiViVax Consortium, a 5 year project which commenced in 2023 working on development of vaccines against P. vivax. Our work within this consortium falls under two main areas. Firstly, we are studying the human antibody responses induced by the PvDBPII based vaccines to determine what antibodies are induced by the vaccines and how these antibodies prevent parasites invading red blood cells. The antibodies are tested for parasite growth inhibition activity in the laboratory using an assay that uses genetically engineered P. knowlesi parasites, which have the P. knowlesi DBP genes replaced with the P. vivax DBP gene. P. knowlesiis a closely related Plasmodium parasite species and unlike P. vivax, is able to be cultured long term in the laboratory. The knowledge gained from studies on vaccine-induced antibody responses will be used to improve the design of next-generation vaccines targeting the blood-stage of the parasite life cycle.
Our other work within the Consortium is focussed on development of a controlled human malaria infection P. vivaxrelapse model in humans. This will run alongside work on pre-clinical and clinical model development and parasite immunobiology by other Consortium partners with the aim to improve our understanding of P. vivax relapse and transmission and provide tools to test interventions against other life cycle stages of the P. vivax parasite.
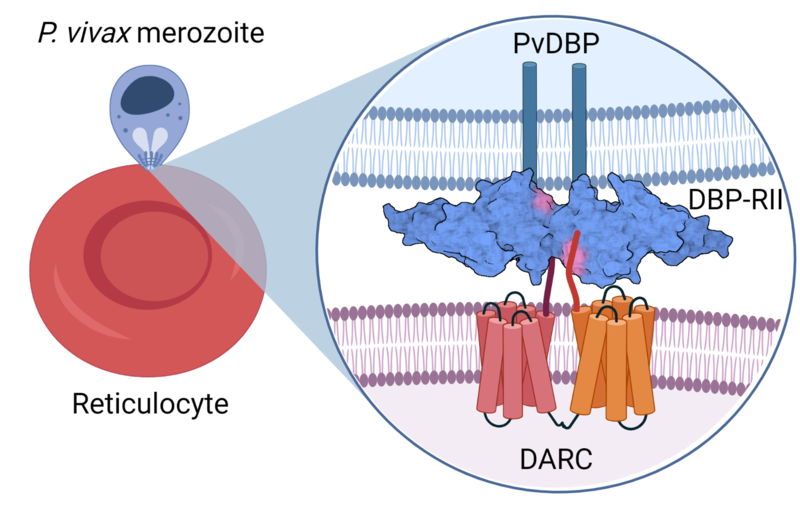
PvDBP-DARC interaction at the merozoite-reticulocyte interface
For more information:
https://optivivax.web.ox.ac.uk/home (website link to Optivivax)
Hou, Mimi M., et al. "Vaccination with Plasmodium Vivax Duffy-Binding Protein Inhibits Parasite Growth during Controlled Human Malaria Infection." Science Translational Medicine (2023).
Payne, Ruth O., et al. "Human vaccination against Plasmodium vivax Duffy-binding protein induces strain-transcending antibodies." JCI Insight (2017).
Rawlinson, Thomas A., et al. "Structural basis for inhibition of Plasmodium vivax invasion by a broadly neutralizing vaccine-induced human antibody." Nature Microbiology (2019).
"Controlled human malaria infection with a clone of Plasmodium vivax with high quality genome assembly." JCI Insight (2021).
">
Human antibodies targeting P. vivax
To replicate, P. vivax must invade immature red blood cells through a process requiring interaction of the P. vivax Duffy binding protein (PvDBP) with its human receptor, the Duffy antigen receptor for chemokines (DARC) on red blood cells. Naturally-acquired antibodies that inhibit this interaction are associated with clinical immunity, making PvDBP a leading candidate for inclusion in a vaccine to prevent P. vivax malaria. We have isolated a panel of monoclonal antibodies from human volunteers in our clinical trials who were immunised with vaccines targeting PvDBP. We are determining the epitopes and binding properties of each mAb. We are also determining the functional ability of the mAbs to inhibit red blood cell invasion against different strains of PvDBP and to prevent PvDBP from binding to its receptor DARC. These findings will guide future vaccine design strategies and open up possibilities for testing the prophylactic use of the most potent mAb that retains inhibitory activity against different strains of PvDBP.
Rawlinson, Thomas A., et al. "Structural basis for inhibition of Plasmodium vivax invasion by a broadly neutralizing vaccine-induced human antibody." Nature Microbiology (2019).
Hou, Mimi M., et al. "Vaccination with Plasmodium Vivax Duffy-Binding Protein Inhibits Parasite Growth during Controlled Human Malaria Infection." Science Translational Medicine (2023).


